Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Fujita, Yoshitaka; Niizeki, Tomotake*; Fukumitsu, Nobuyoshi*; Ariga, Katsuhiko*; Yamauchi, Yusuke*; Malgras, V.*; Kaneti, Y. V.*; Liu, C.-H.*; Hatano, Kentaro*; Suematsu, Hisayuki*; et al.
Bulletin of the Chemical Society of Japan, 95(1), p.129 - 137, 2022/01
Times Cited Count:8 Percentile:76.16(Chemistry, Multidisciplinary)In this work, the mechanisms responsible for the adsorption of molybdate ions on alumina are investigated using in-depth surface analyses carried out on alumina specimens immersed in solutions containing different molybdate ions at different pH values. The obtained results reveal that when alumina is immersed in an acidic solution containing molybdate ions, the hydroxyl groups present on the surface are removed to generate positively charged sites, and molybdate ions (MoO
 or AlMo
or AlMo O
O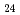 H
H
 ) are adsorbed by electrostatic interaction. Alumina dissolves slightly in an acidic solution to form AlMo
) are adsorbed by electrostatic interaction. Alumina dissolves slightly in an acidic solution to form AlMo O
O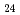 H
H
 , which is more easily desorbed than MoO
, which is more easily desorbed than MoO
 . Furthermore, the enhancement in the Mo adsorption or desorption property may be achieved by enriching the surface of the alumina adsorbent with many -OH groups and optimizing Mo solution to adsorb molybdate ions on alumina as MoO
. Furthermore, the enhancement in the Mo adsorption or desorption property may be achieved by enriching the surface of the alumina adsorbent with many -OH groups and optimizing Mo solution to adsorb molybdate ions on alumina as MoO
 ions. These findings will assist researchers in engineering more efficient and stable alumina-based adsorbents for molybdenum adsorption used in medical radioisotope (
ions. These findings will assist researchers in engineering more efficient and stable alumina-based adsorbents for molybdenum adsorption used in medical radioisotope ( Mo/
Mo/ Tc) generators.
Tc) generators.
Kato, Chiaki
Comprehensive Nuclear Materials, 2nd Edition, Vol.4, p.528 - 563, 2020/08
In spent fuel reprocessing plants, various nitric media are encountered throughout the PUREX process, used in the separation of fission products, uranium, and plutonium. The PUREX process is thus highly corrosive as it takes place at high temperatures under high concentrations of nitric acid solution containing oxidizing metal ions from spent fuel. In this review, the unique chemical properties of nitric acid are first described. Secondly, the process of oxidizing power generation in boiling nitric acid under heat transfer is described using the redox potential and a thermodynamic model of boiling nitric acid. Finally, the corrosion behavior and corrosion acceleration mechanism specific to the reprocessing environments are described from the perspective of solution chemistry.
Ueno, Fumiyoshi; Irisawa, Eriko; Kato, Chiaki; Igarashi, Takahiro; Yamamoto, Masahiro; Abe, Hitoshi
Proceedings of European Corrosion Congress 2016 (EUROCORR 2016) (USB Flash Drive), 7 Pages, 2016/09
In this study, we focused on the effect of the boiling of nitric acid solution on the corrosion of a stainless steel-made concentrator in reduced pressure in fuel reprocessing plant. In order to perform the simulation test in a non-radioactive condition, nitric acid solution with the addition of vanadium as an oxidizing metal ion were used. Corrosion tests were carried out under the conditions of boiling at reduced pressure, and of non-boiling at normal pressure and several temperatures. As a result, corrosion was accelerated by the solution boiling while it was not by non-boiling at the same temperature. It was found also that the temperature dependence of corrosion rate is the same in the both conditions of boiling and non-boiling. The corrosion accelerating effect will be discussed on the basis of the reaction among nitric acid, NOx and vanadium, etc.
Irisawa, Eriko; Ueno, Fumiyoshi; Kato, Chiaki; Abe, Hitoshi
Zairyo To Kankyo, 65(4), p.134 - 137, 2016/04
In order to investigate the effect of boiling under reduced pressure on corrosion of stainless steel in the nitric acid solution, the corrosion tests simulating the high-level radioactive liquid waste evaporator were performed. The results of immersion tests of stainless steels in the solution with and without boiling showed that the corrosion rates in boiling solution were larger than those in not boiling solution in case of same temperature of solution. Moreover, the cathode polarization curves showed that the corrosion potential of stainless steel in boiling solutions were shifted nobler, and the current intensity became larger than that in not boiling solutions. According to these results, it can be concluded that boiling of solution under reduced pressure accelerate the corrosion rates.
Chiba, Atsuya; Uno, Sadanori; Okoshi, Kiyonori; Yamada, Keisuke; Saito, Yuichi; Ishii, Yasuyuki; Sakai, Takuro; Sato, Takahiro; Mizuhashi, Kiyoshi
JAEA-Review 2005-001, TIARA Annual Report 2004, p.358 - 360, 2006/01
no abstracts in English
Wasikiewicz, J. M.; Nagasawa, Naotsugu; Tamada, Masao; Mitomo, Hiroshi*; Yoshii, Fumio
Nuclear Instruments and Methods in Physics Research B, 236(1-4), p.617 - 623, 2005/07
Times Cited Count:54 Percentile:95.02(Instruments & Instrumentation)The absorption ability of various metal ions into EB - radiation crosslinked carboxymethylchitin and carboxymethylchitosan has been investigated. The highest adsorption of Scandium and Gold has been obtained for carboxymethylchitin (CMCht) and carboxymethylchitosan (CMChts), respectively. Kinetic studies showed that adsorption of most of the metal ions occur in a relatively short period of time (2 hours). Detail investigation of adsorption of gold ions has been carried out for both hydrogels. The maximum uptake of Au cations, based on Langmuir equation was determined to be 37.59 mg/g for CMChts and 11.86 mg/g for CMCht. Both hydrogels indicate favorable adsorption of gold cations.
Nakanoya, Takamitsu; Matsuda, Makoto; Fujii, Yoshio*
Proceedings of 2nd Annual Meeting of Particle Accelerator Society of Japan and 30th Linear Accelerator Meeting in Japan, p.729 - 730, 2005/07
TRIAC (Tokai Radioactive Ion Accelerator Complex) facility can accelerate both radioactive and stable ion beams up to 1.1Mev/u. In this facility, an ECR ion source is used for production of stable ion beams. Stable ion beams are used mainly as a pilot beam for radioactive ion beam. ECR ion source is required to produce many kinds of ion species, not only gas state elements. So, we developed a high temperature oven for obtaining metal ion beams. This paper describes the design and detail of the oven and experimental results.
Hendri, J.*; Hiroki, Akihiro*; Maekawa, Yasunari; Yoshida, Masaru; Katakai, Ryoichi*
Radiation Physics and Chemistry, 60(6), p.617 - 624, 2001/03
Times Cited Count:33 Percentile:89.5(Chemistry, Physical)no abstracts in English
Yokota, Wataru; Arakawa, Kazuo; Okumura, Susumu; Fukuda, Mitsuhiro; Kamiya, Tomihiro; Nakamura, Yoshiteru
Proceedings of 4th International Workshop on Radiation Effects on Semiconductor Devices for Space Application, p.179 - 184, 2000/10
no abstracts in English
J. A. BERRY*; M. BROWNSWORD*; D. J. ILETT*; Linklater, C. M.*; Mason, C.*; TWEED, C. J.*
JNC TJ8400 2000-060, 60 Pages, 2000/02
Batch sorption experiments have been carried out to investigate the sorption behaviour of plutonium onto basalt and sandstone from the appropriate rock-equilibrated waters under different redox eonditions. Redox Potentials in solution were controlled by the addition of two reducing agents and one oxidising agent. Thermodynamic chemical modelling was undertaken to interpret the results. The sorption models were based on iron oxide. They adequately reproduced the data for sorption of plutonium onto sandstone, but tended to underpredict sorption onto basalt.
Tochiyama, Osamu*
JNC TJ8400 2000-044, 53 Pages, 2000/02
To estimate the polyelectrolyte effect and the effect of the heterogeneous composition of humic acids, the complex formation constants of Eu(III) and Ca(II) with Aldrich humic acid and polyacrylic acid were obtained, for Eu(10 to 10
to 10 M) by solvent extraction with TTA and TBP in xylene, for Ca (10
M) by solvent extraction with TTA and TBP in xylene, for Ca (10 M) with TTA and TOPO in cyclohexane and for Ca(10
M) with TTA and TOPO in cyclohexane and for Ca(10 M) by using ion-selective electrode. By defining the apparent formation as
M) by using ion-selective electrode. By defining the apparent formation as  = [MR
= [MR ]/([M][R]), where [R] denotes the concentration of dissociated functional group, [M] and [MR
]/([M][R]), where [R] denotes the concentration of dissociated functional group, [M] and [MR ] denote the concentration of free and bound metal ion and pcH is defined as-log[H], the values of log
] denote the concentration of free and bound metal ion and pcH is defined as-log[H], the values of log have been obtained at pcH 4.8 - 5.5 in 0.1 - 1.0M NaClO
have been obtained at pcH 4.8 - 5.5 in 0.1 - 1.0M NaClO and NaCl. Log
and NaCl. Log of Eu-humate varied from 5.0 to 9.3 and that of Ca-humate from 2.0 to 3.4..For both humate and polyacrylate, log
of Eu-humate varied from 5.0 to 9.3 and that of Ca-humate from 2.0 to 3.4..For both humate and polyacrylate, log increased with pcH or with the degree of dissociation. The increase in the ionic strength O.1 to 1.0 M decreased the log
increased with pcH or with the degree of dissociation. The increase in the ionic strength O.1 to 1.0 M decreased the log , the decrease in log
, the decrease in log of Eu(III)-humate is 1.6, that of Eu(III), polyacrylate 0.7, that of Ca(II)-humate 1.9 and that of Ca(II)-polyacrylate 1.2. While the increase in the metal ion produced no effect on log
of Eu(III)-humate is 1.6, that of Eu(III), polyacrylate 0.7, that of Ca(II)-humate 1.9 and that of Ca(II)-polyacrylate 1.2. While the increase in the metal ion produced no effect on log of polyacrylate, log
of polyacrylate, log of humate decreased. Depending on the concentration of Eu(III), the coexistence of Ca(II) reduced log
of humate decreased. Depending on the concentration of Eu(III), the coexistence of Ca(II) reduced log  of humate by 0 to 0.8. The dependence of log
of humate by 0 to 0.8. The dependence of log of humate on the metal ion concentration suggests the coexistence of strong and weak binding sites in the hmnic acid.
of humate on the metal ion concentration suggests the coexistence of strong and weak binding sites in the hmnic acid.
Matsuda, Makoto; Takeuchi, Suehiro; Kobayashi, Chiaki*
KEK Proceedings 99-22, p.17 - 27, 2000/01
no abstracts in English
Yokota, Wataru; Saito, Yuichi; Nara, Takayuki; Ishii, Yasuyuki; Okoshi, Kiyonori; Arakawa, Kazuo
Proc. of 14th Int. Workshop on ECR Sources (ECRIS99), p.172 - 175, 1999/00
no abstracts in English
Kato, Toshihiro*; ; ; ; Ishibashi, Yuzo; Takeda, Seiichiro
PNC TN8410 98-070, 31 Pages, 1998/02
None
 plasma
plasmaSaito, Yuichi; Okoshi, Kiyonori; Yokota, Wataru
Review of Scientific Instruments, 69(2), p.703 - 705, 1998/02
Times Cited Count:6 Percentile:49.2(Instruments & Instrumentation)no abstracts in English
Kimura, Takaumi; Kato, Yoshiharu; Yoshida, Zenko
Journal of Nuclear Science and Technology, 34(7), p.717 - 719, 1997/07
Times Cited Count:1 Percentile:14.48(Nuclear Science & Technology)no abstracts in English